Chemical Reaction And Chemical Equation
1. A compound ‘A’ is used in the manufacture of cement. When dissolved in water, it evolves a large amount of heat and forms compound ‘B’.
(i) Identify A and B.(ii) Write chemical equation for the reaction of A with water.
(iii) List two types of reaction in which this reaction may be classified. (2020)
Answer:
(i) A is calcium oxide, CaO which is used in the manufacturing of cement.
B is calcium hydroxide Ca(OH)3.
(iii) The given reaction is a combination reaction.
Example : NH3(g)(g) + HCl(g) → NH4Cl(s)
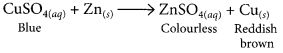
2. Mention with reason the colour changes observe when:
(i) silver chloride is exposed to sunlight.(ii) copper powder is strongly heated in the presence of oxygen.
(iii) a piece of zinc is dropped in copper sulphate solution.
Ans: (i) When white silver chloride is left exposed to sunlight, its colour changes to grey as it decomposes to silver in the presence of sunlight.
This type of reaction is called photodecomposition reaction.
(ii) When copper powder is strongly heated in presence of oxygen, the reddish brown surface of copper powder becomes coated with a black substance which is copper oxide.
3. Lead nitrate solution is added to a test tube containing potassium iodide solution.
(a) Write the name and colour of the compound precipitated.
(b) Write the balanced chemical equation for the reaction involved.
(c) Name the type of this reaction justifying your answer.
Ans:
(a) When lead nitrate is added to potassium iodide then yellow precipitate of lead iodide is formed along with potassium nitrate.
(b) Balanced chemical reaction is as follows
(a) When lead nitrate is added to potassium iodide then yellow precipitate of lead iodide is formed along with potassium nitrate.
(b) Balanced chemical reaction is as follows
(c) This type of reaction is called precipitation reaction in which one of the products formed is an insoluble substance or this is also called double displacement reaction
4. unsolved question..
Mention the type of chemical reaction that takes place when:
(i) a magnesium ribbon is burnt in air.
(ii) limestone is heated.
(iii) silver bromide is exposed to sunlight.
(iv) electricity is passed through acidified water.
(v) ammonia and hydrogen chloride are mixed with each other.
Write the chemical equation for each reaction
(i) a magnesium ribbon is burnt in air.
(ii) limestone is heated.
(iii) silver bromide is exposed to sunlight.
(iv) electricity is passed through acidified water.
(v) ammonia and hydrogen chloride are mixed with each other.
Write the chemical equation for each reaction
try to give answers of these...soon i will share its answers.



Comments
Post a Comment